Chemical Change of a Candle: What Really Happens When a Candle Burns
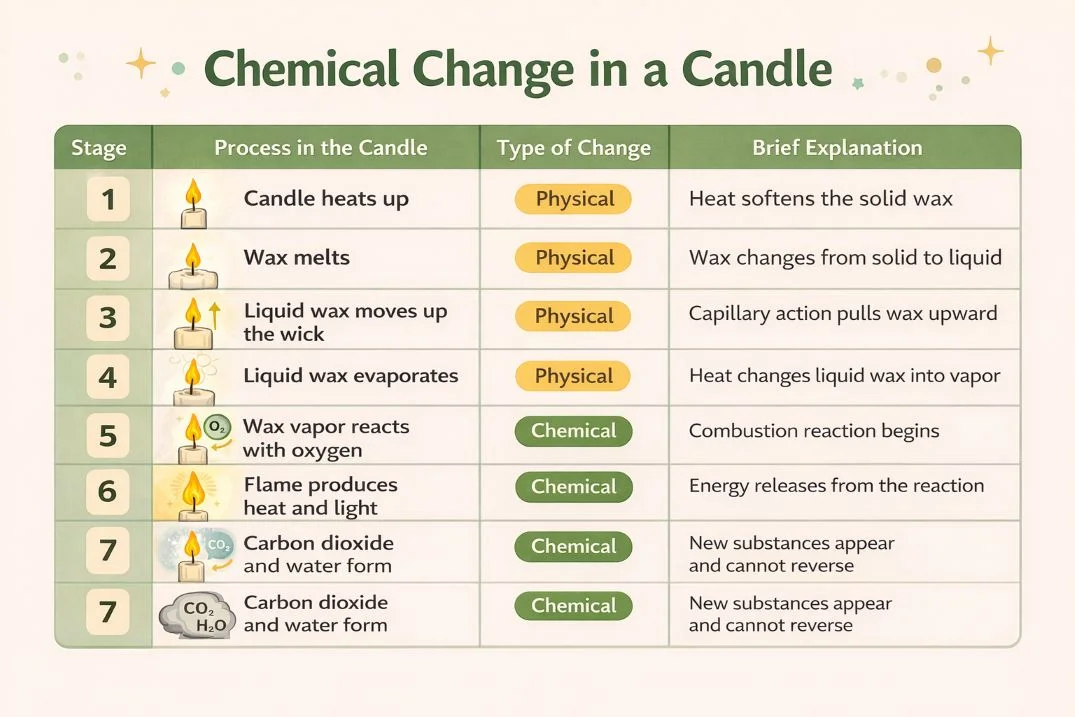
First of all, the chemical change of a candle involves far more than simple melting or a visible flame. In fact, when a candle burns, it immediately starts a controlled chemical reaction that transforms wax into energy and new substances. As a result, the candle produces light, heat, and gases while the wax slowly disappears. Therefore, understanding this chemical change helps explain how everyday combustion works in a clear and practical way.
Why Candle Burning Is a Chemical Change
To begin with, a chemical change always creates new substances. During candle burning, Paraffin wax does not only change shape or state. Instead, wax vapor reacts with oxygen and forms carbon dioxide, water vapor, heat, and light. Consequently, these products differ completely from the original wax. Because the process creates new materials and cannot reverse, candle burning clearly represents a chemical change.
Table of Contents
ToggleRole of Wax in the Chemical Change of a Candle
First, candle wax consists mainly of hydrocarbon molecules that store chemical energy. Then, when the candle burns, the flame breaks the chemical bonds inside these molecules and releases energy. Importantly, the wax itself does not burn as a solid or liquid. Instead, heat from the flame turns liquid wax into vapor, and afterward this vapor reacts with oxygen. As a result, this reaction keeps the flame active and continuously drives the chemical change forward.
Energy Conversion During Candle Burning
Next, the chemical change of a candle involves clear energy conversion. Initially, chemical energy inside the wax changes into heat and light. Consequently, heat warms the surrounding air, while light provides illumination. Therefore, this steady release of energy clearly shows how chemical energy transforms into usable forms during combustion.
Chemical Reaction Behind the Flame
At this stage, the main reaction during candle burning follows basic combustion principles. Specifically, wax vapor acts as the fuel, oxygen from the air supports the reaction, and carbon dioxide and water vapor form as products. As long as oxygen and wax vapor remain available, the reaction continues. However, removing either element immediately stops the flame and ends the chemical change.
Why Melting Alone Is Not a Chemical Change
Many people mistakenly confuse melting with burning. However, melting represents a physical change because wax only changes from solid to liquid. Moreover, cooling can easily reverse this process. In contrast, the chemical change starts only when wax vapor reacts with oxygen. At that point, new substances form, and therefore the process cannot reverse.
Factors That Affect the Chemical Change of a Candle
Several factors directly influence how efficiently a candle undergoes chemical change. For example, oxygen supply affects flame strength. In addition, wick size controls how much fuel reaches the flame. Furthermore, wax composition influences burn stability. Finally, air movement around the flame affects combustion quality. Consequently, proper balance between these factors produces a clean and stable flame.
Scientific Importance of Candle Combustion
Because of its simplicity, scientists often use candle flames to study combustion, heat transfer, and reaction behavior. Moreover, engineers apply these same principles when designing burners, engines, and energy systems. Therefore, the candle flame provides a simple yet powerful example of chemical change in action.
Conclusion
In summary, the chemical change of a candle transforms wax into energy and new substances through combustion. First, wax vapor reacts with oxygen. Then, the reaction releases heat and light and forms carbon dioxide and water vapor. Unlike melting, this process cannot reverse. Therefore, by understanding this chemical change, we gain a clearer view of energy conversion and basic chemical reactions found in everyday life.